
How to develop the right operating model when transitioning from clinical development to the commercial stage? This critical phase for emerging biotech and pharma companies demands the establishment of robust pharmacovigilance (PV) systems. Given the resource and expertise constraints often faced by smaller firms, outsourcing to Contract Research Organizations (CROs) or specialized PV service providers emerges as a viable solution. However, this approach introduces its own set of challenges, including maintaining control, ensuring quality, and managing flexibility.
Why is that important?
Transitioning from a GCP-focused clinical development stage to a GVP-driven commercial (“post-marketing”) phase is a challenge that hinges on strategic decisions around the operating model for pharmacovigilance activities. This transition requires not only a shift towards comprehensive safety monitoring but also a critical evaluation of how to structure the post-marketing surveillance operations to ensure efficiency, compliance, and scalability.
The most notable shift is the expansion of the drug safety horizon from what it was in the distinct clinical setting. The post-marketing PV system expands beyond the R&D barrier well into other fields like commercial activities.
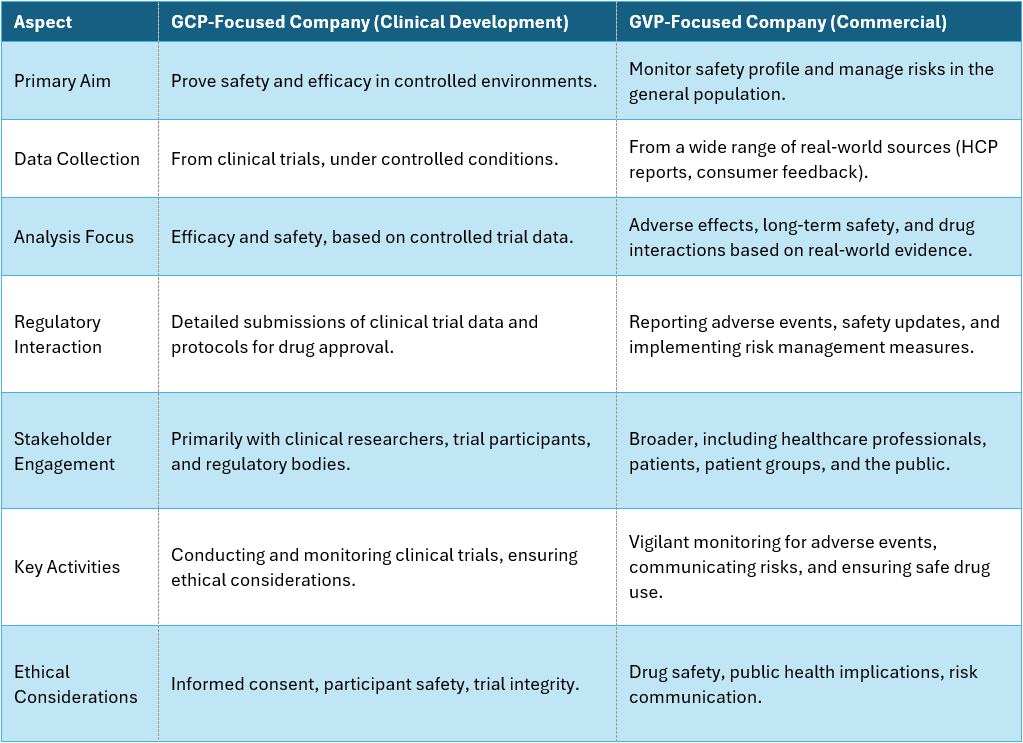
Table 1: Differences of a GCP- to a GVP-focused organization. While both perspectives can exist in the same company, the transition process can be challenging.
Determining the right Operating Model
Determining the optimal operating model - whether to manage pharmacovigilance activities in-house, outsource to Contract Research Organizations (CROs), adopt a hybrid approach, or explore options like labor leasing - is a strategic decision that impacts the organization’s agility, control over pharmacovigilance processes, and ultimately, the ability to ensure patient safety.
Figure 1: Example for different sourcing models, applicable for most areas within an organization
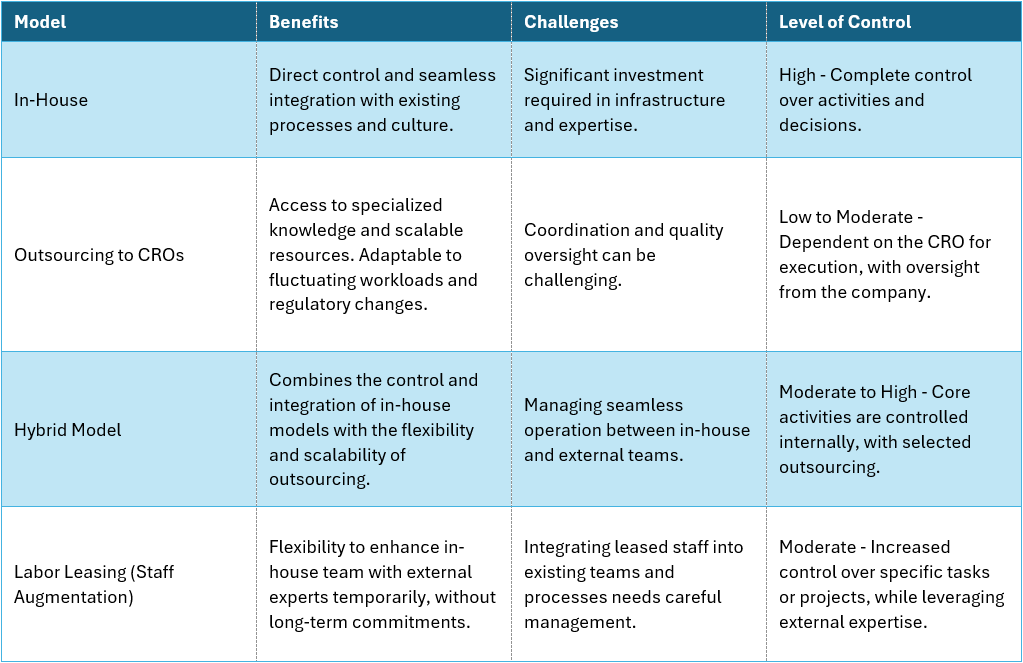
Table 2: Example for different sourcing models, applicable for most areas within an organization
For growing companies, creating a heavily outsourced PV organization is a double-edged sword. On the one hand, it offers access to essential resources and expertise, crucial to cover all requirements without building a large internal overhead.
“Just handing over” the entirety of operations to a service provider on the other hand is most certainly a mistake for biotech and pharma companies, particularly when dealing with the critical transition from late-stage clinical trials to post-marketing pharmacovigilance. The right operating model involves not just selecting a competent CRO but also defining what operations and responsibilities should be outsourced and what should remain in-house as strategic core competencies.
Developing an adequate operating strategy for pharmacovigilance goes beyond just improving efficiency and managing costs. The key is a strategic approach that encompasses asking the right questions, selecting the right partners, and maintaining strong oversight.
Developing an adequate operating strategy for pharmacovigilance
That means:
- Distinguish Between Core and Supporting Activities: Decide which PV functions are critical to keep in-house for strategic oversight and which can be efficiently outsourced.
- Align Outsourcing with Strategic Goals: Clearly identify how outsourcing fits into the company’s broader objectives and complies with regulatory demands.
- Select Partners Carefully: Choose a service provider not just based on cost but for their alignment with the company’s vision, commitment to quality, and operational compatibility.
- Ensure Strong Oversight: Implement effective quality management and oversight processes to guarantee compliance, maintain data integrity, and remain flexible to new developments.
Adopting these strategies enables organizations to manage the balance between using external expertise and keeping necessary control. This approach not only ensures compliance and safety but also positions companies for resilience and growth in a competitive environment.
From GCP to GVP: A Pharmacovigilance Guide for Emerging Biotech Firms
Are you interested to learn more about that topic?
- Discover how these challenges can be met head-on,
- the implications of outsourcing for pharmacovigilance activities, and
- the key elements required for a robust and adaptable operational structure.
Image source: Unsplash














